Don’t Put Rotator Cuff Surgery Off Anymore!
The REGENETEN Bioinductive Implant, an innovative, minimally invasive solution, goes beyond traditional surgery to help the tendon heal through the induction of new tendinous tissue growth1,2,3.
The REGENETEN Bioinductive Implant is an innovative technology designed to address the treatment of rotator cuff disease
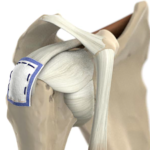 The REGENETEN Bioinductive Implant offers a new solution that supports the body’s natural healing response to facilitate new tissue growth and potentially disrupt disease progression 1,2,7-9 by providing a framework for tissue growth.
The REGENETEN Bioinductive Implant offers a new solution that supports the body’s natural healing response to facilitate new tissue growth and potentially disrupt disease progression 1,2,7-9 by providing a framework for tissue growth.
What is it?
The technology includes a collagen based bioinductive implant about the size of a postage stamp. This implant is delivered arthroscopically through a small incision over the location of your rotator cuff tendon injury. Your physician will secure it in place with small anchors.
The REGENETEN Bioinductive Implant is an innovative technology designed to address the treatment of rotator cuff disease
 The REGENETEN Bioinductive Implant offers a new solution that supports the body’s natural healing response to facilitate new tissue growth and potentially disrupt disease progression 1,2,7-9 by providing a framework for tissue growth.
The REGENETEN Bioinductive Implant offers a new solution that supports the body’s natural healing response to facilitate new tissue growth and potentially disrupt disease progression 1,2,7-9 by providing a framework for tissue growth.
What is it?
The technology includes a collagen based bioinductive implant about the size of a postage stamp. This implant is delivered arthroscopically through a small incision over the location of your rotator cuff tendon injury. Your physician will secure it in place with small anchors.
Patient Testimonial
How bad is your shoulder pain?
Answer these common questions to help your doctor better understand your shoulder pain.
Surgeons Can Address Rotator Cuff Injury Sooner.
Watch the videos below to hear how surgeons are using the REGENETEN Bioinductive Implant to address rotator cuff disease.
A new approach – REGENETEN Bioinductive Implant
Until now, surgeons have focused on addressing the mechanics of the shoulder when repairing a rotator cuff injury, but had limited options to address the biology of healing. Traditional rotator cuff repair procedures involve suturing tendon to bone are associated with potentially long rehabilitation and substantial lifestyle interruption. As a result, many people choose to forego surgery until their pain is severe and everyday tasks are affected. However, as rotator cuff disease progresses, it can become increasingly difficult for your surgeon to repair and small tears can grow in severity and size over time 4,5,6.

How does the REGENETEN Bioinductive Implant benefit you?
No matter where you are in the progression of rotator cuff disease, the REGENETEN Bioinductive Implant has shown consistent healing of rotator cuff tears in both partial and full thickness tears 1,2,8,12.
- Preliminary evidence indicates a reduction in re-tear rates compared to the standard of care in patients with full thickness tears 3,13
- No tear progression was observed by MRI in any patients at 24 months in a study on partial thickness tears (n=13) 1
- Clinically meaningful improvements in pain and function scores at one year compared to pre-operation values 14,15
- The REGENETEN Bioinductive Implant has been shown to be a safe and effective solution in the treatment of rotator cuff disease. 1,2,7,11,12
References
- Bokor DJ, Sonnabend D, Deady L, et al. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up. Muscles, Ligaments Tendons J 2016;6(1):16-25.
- Schlegel TF, Abrams JS, Bushnell BD, Brock JL, Ho CP. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: a prospective multicenter study. J Shoulder Elbow Surg. 2018 27(2):242-251.
- Bokor DJ, Sonnabend D, Deady L, et al. Preliminary investigation of a biological augmentation of rotator cuff repairs using a collagen implant: a 2-year MRI follow-up. Muscles, Ligaments Tendons J 2015;5(3):144-150.
- Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: A systematic review. Arthroscopy. 2015;31(12):2472-80.
- Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: A prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
- Heuberer PR, Smolen D, Pauzenberger L et al. Longitudinal long-term magnetic resonance imaging and clinical follow-up after single-row arthroscopic rotator cuff repair. Am J Sports Med. 2017;45(6):1283-1288.
- Van Kampen C, Arnoczky S, et al. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: a histological evaluation in sheep. Muscles, Ligaments Tendons J. 2013;3(3):229-235.
- Bokor DJ, Sonnabend DH, et al. healing of partial thickness rotator cuff tears following arthroscopic augmentation with a highly porous collagen implant: a 5-year clinical and MRI follow-up. Muscles, Ligaments Tendons J 2019;9(3):338-347.
- McElvany MD, McGoldrick, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43(2)491-500.
- Smith + Nephew 2020 REGENETEN Collagen Implant Physical Characteristics. Internal Report
- Arnoczky SP, Bishai SK, Schofield B, et al. Histologic Evaluation of Biopsy Specimens Obtained After Rotator Cuff Repair Augmented With a Highly Porous Collagen Implant. Arthroscopy. 2017;33(2):278-283.
- Thon SG, O’Malley L, O’Brien MJ, Savoie FH. Evaluation of Healing Rates and Safety With a Bioinductive Collagen Patch for Large and Massive Rotator Cuff Tears: 2-Year Safety and Clinical Outcomes. Am J Sports Med 2019;47(8):1901-1908.
- Smith and Nephew 2019. An overview of the outcomes associated with the standard of care for the surgical treatment of rotator cuff tears. Internal Report EO/SPM/REGENETEN/005/v1
- McIntyre L, Bishai SK, et al. Patient-reported outcomes following use of a bioabsorbable collagen implant to treat partial and full-thickness rotator cuff tears. Arthroscopy. 201935(8):2262-2271.
- Cvetanovich GL, Gowd AK, et al. Establishing clinically significant outcome after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2019;28(5):939-948.
*on human biopsy (n=1) and in-vivo sampling


